Hydrogen for fuel cells is made through methods like steam methane reforming and water electrolysis, with a growing shift toward green hydrogen powered by renewable energy. This clean fuel produces only water as a byproduct, making it a key player in the transition to sustainable transportation and industry.
Key Takeaways
- Steam Methane Reforming (SMR) is the most common method: It uses natural gas and steam to produce hydrogen, but emits CO₂ unless paired with carbon capture.
- Electrolysis splits water into hydrogen and oxygen: When powered by renewables like wind or solar, it creates “green hydrogen” with zero emissions.
- Green hydrogen is the future of clean energy: It supports decarbonization in transport, industry, and power generation without fossil fuel dependence.
- Emerging technologies include biomass gasification and photoelectrochemical processes: These offer sustainable alternatives but are still in development.
- Hydrogen purity is critical for fuel cells: Impurities can damage fuel cell components, so high-purity production and storage are essential.
- Infrastructure and cost remain challenges: Scaling up production and building delivery networks are key to widespread adoption.
- Government policies and investments are accelerating innovation: Countries like Germany, Japan, and the U.S. are leading the charge in hydrogen development.
📑 Table of Contents
- Introduction: The Rise of Hydrogen as a Clean Energy Solution
- Why Hydrogen Matters for Fuel Cells
- Steam Methane Reforming: The Current Workhorse
- Electrolysis: The Path to Green Hydrogen
- Other Methods: Biomass, Solar, and Beyond
- Purification and Storage: Getting Hydrogen Ready for Use
- The Future of Hydrogen Production
- Conclusion: A Cleaner Future Powered by Hydrogen
Introduction: The Rise of Hydrogen as a Clean Energy Solution
Imagine a world where cars, buses, and even industrial plants run on a fuel that emits nothing but water vapor. That’s not science fiction—it’s the promise of hydrogen fuel cells. These devices convert hydrogen gas into electricity through a clean electrochemical process, powering everything from smartphones to city buses. But here’s the catch: to truly be “clean,” the hydrogen itself must be produced sustainably.
Hydrogen is the most abundant element in the universe, but it doesn’t exist freely on Earth. It’s always bound to other elements—like oxygen in water (H₂O) or carbon in methane (CH₄). So, to use hydrogen as a fuel, we must extract it from these compounds. This process, known as hydrogen production, is the foundation of the hydrogen economy. And as the world races to cut carbon emissions, understanding how hydrogen is produced for fuel cells has never been more important.
Why Hydrogen Matters for Fuel Cells
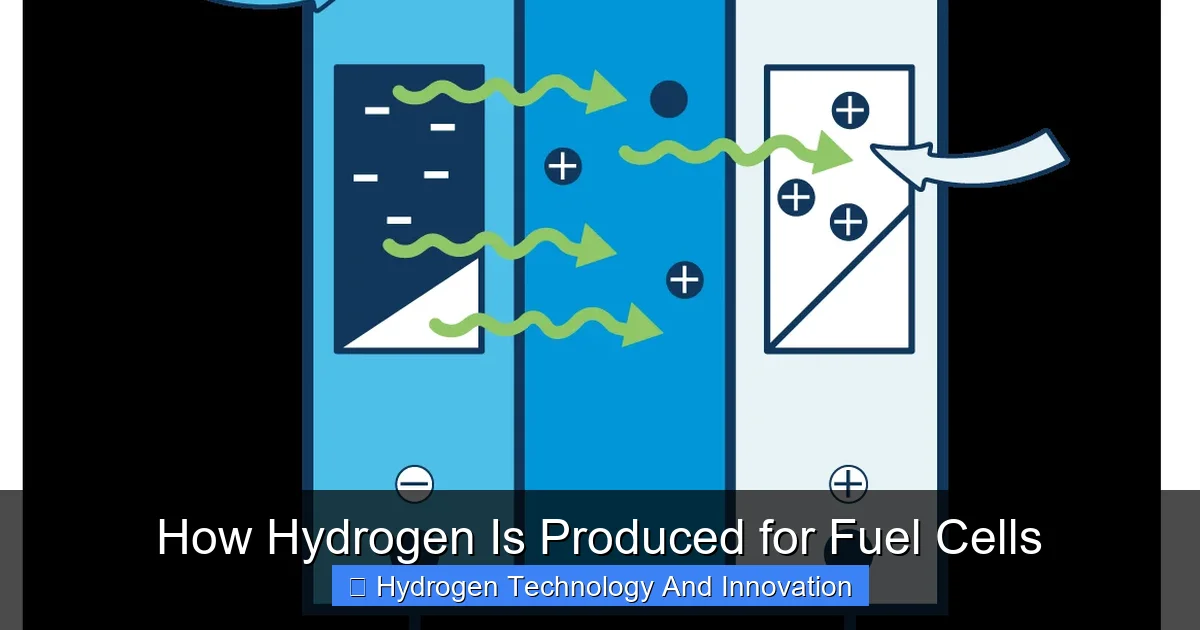
Visual guide about How Hydrogen Is Produced for Fuel Cells
Image source: eia.gov
Fuel cells are like batteries that never run out—as long as you keep feeding them hydrogen and oxygen. They’re quiet, efficient, and produce zero tailpipe emissions. That’s why they’re being adopted in heavy-duty trucks, forklifts, backup power systems, and even submarines. But the environmental benefit hinges on how the hydrogen is made.
If hydrogen comes from fossil fuels without carbon capture, it’s not much cleaner than gasoline. But if it’s made using renewable energy, it becomes a truly green fuel. This distinction is why the method of production matters just as much as the end use. The goal is to move from “gray” and “blue” hydrogen (made from fossil fuels) to “green” hydrogen (made from water and clean electricity).
Steam Methane Reforming: The Current Workhorse
How SMR Works
Today, about 95% of the world’s hydrogen is produced using steam methane reforming (SMR). This method starts with natural gas—mostly methane (CH₄)—and reacts it with high-temperature steam (around 700–1000°C) in the presence of a catalyst, usually nickel. The reaction produces hydrogen (H₂) and carbon monoxide (CO). A second step, called the water-gas shift reaction, converts the CO and more steam into additional hydrogen and carbon dioxide (CO₂).
The chemical reactions look like this:
– CH₄ + H₂O → CO + 3H₂ (steam reforming)
– CO + H₂O → CO₂ + H₂ (water-gas shift)
After these steps, the hydrogen is separated from the CO₂ and other gases using pressure swing adsorption (PSA) or membrane separation, resulting in high-purity hydrogen ready for fuel cells.
Pros and Cons of SMR
SMR is efficient and cost-effective, producing hydrogen at around $1–2 per kilogram. It’s the reason hydrogen is already used in oil refining, ammonia production, and food processing. But it’s not clean. Each kilogram of hydrogen made this way releases about 9–12 kilograms of CO₂ into the atmosphere.
That’s why SMR is often called “gray hydrogen.” To reduce its climate impact, some facilities are adding carbon capture and storage (CCS), turning it into “blue hydrogen.” With CCS, up to 90% of the CO₂ can be captured and stored underground. While better than gray, blue hydrogen still relies on fossil fuels and isn’t a long-term solution.
Real-World Example: Industrial Hydrogen Plants
One of the largest SMR plants in the world is in Alberta, Canada, operated by Air Products. It produces over 300 million standard cubic feet of hydrogen per day—enough to fuel thousands of fuel cell vehicles. The plant uses natural gas and has invested in carbon capture technology to reduce emissions. Similar facilities exist in the U.S. Gulf Coast, where abundant natural gas and existing infrastructure make SMR economically attractive.
Electrolysis: The Path to Green Hydrogen
How Electrolysis Splits Water
Electrolysis is a cleaner alternative. It uses electricity to split water (H₂O) into hydrogen and oxygen. The process happens in an electrolyzer, which contains two electrodes (an anode and a cathode) separated by an electrolyte. When electricity is applied, water molecules break apart: hydrogen gas forms at the cathode, and oxygen gas forms at the anode.
There are three main types of electrolyzers:
– Alkaline Electrolyzers: Use a liquid alkaline solution (like potassium hydroxide) as the electrolyte. They’re mature technology, reliable, and cost-effective, but less flexible in operation.
– Proton Exchange Membrane (PEM) Electrolyzers: Use a solid polymer membrane and can respond quickly to variable power inputs, making them ideal for pairing with solar or wind energy.
– Solid Oxide Electrolyzers (SOEC): Operate at high temperatures (700–850°C) and are highly efficient, especially when paired with waste heat from industrial processes.
Green Hydrogen: Powered by Renewables
The real magic happens when electrolysis is powered by renewable energy. Wind turbines, solar panels, or hydropower generate electricity with zero emissions. When that electricity splits water, the resulting hydrogen is called “green hydrogen.” It’s the cleanest form of hydrogen available today.
Green hydrogen production is growing rapidly. In 2023, the global capacity for electrolyzers reached over 10 gigawatts (GW), with projections to exceed 100 GW by 2030. Countries like Germany, Australia, and Chile are investing billions in green hydrogen projects, aiming to export it to energy-hungry regions.
Example: The HyDeal Ambition Project
One of the most ambitious green hydrogen initiatives is HyDeal Ambition, a consortium spanning Spain, France, and Portugal. The project plans to build a 67-gigawatt solar-powered hydrogen hub by 2030, producing hydrogen at under $1.50 per kilogram—competitive with fossil-based hydrogen. The hydrogen will be transported via pipeline to industrial users and fueling stations across Europe.
Other Methods: Biomass, Solar, and Beyond
Biomass Gasification
Biomass gasification is another way to produce hydrogen sustainably. It involves heating organic materials—like wood chips, agricultural waste, or algae—in a low-oxygen environment. The heat breaks down the biomass into a mixture of gases, including hydrogen, carbon monoxide, and methane. This “syngas” can then be processed to extract pure hydrogen.
This method is considered carbon-neutral because the CO₂ released during gasification is roughly equal to the CO₂ absorbed by the plants during growth. However, it requires large amounts of biomass and careful management to avoid deforestation or competition with food crops.
Photoelectrochemical (PEC) Water Splitting
Still in the research phase, PEC water splitting uses sunlight to directly split water into hydrogen and oxygen, mimicking photosynthesis. Special semiconductor materials absorb sunlight and generate the electrical potential needed for electrolysis—all in one step.
While promising, PEC systems face challenges in efficiency, durability, and cost. Scientists are working to develop materials that are both highly efficient and stable under long-term exposure to sunlight and water.
Thermochemical Water Splitting
This method uses high-temperature heat (often from nuclear reactors or concentrated solar power) to drive a series of chemical reactions that split water. It doesn’t require electricity, making it potentially more efficient for large-scale production. However, it’s complex and still in the experimental stage.
Purification and Storage: Getting Hydrogen Ready for Use
Why Purity Matters
Fuel cells are sensitive to impurities. Even tiny amounts of sulfur, carbon monoxide, or ammonia can poison the catalyst and reduce performance. That’s why hydrogen used in fuel cells must meet strict purity standards—typically 99.97% pure or higher.
After production, hydrogen is purified using techniques like pressure swing adsorption (PSA), membrane separation, or cryogenic distillation. PSA is the most common, using adsorbent materials to trap impurities while allowing hydrogen to pass through.
Storage and Transportation Challenges
Hydrogen is the smallest and lightest molecule, making it tricky to store and transport. It can leak easily, embrittle metals, and requires high pressure or very low temperatures to become dense enough for practical use.
Common storage methods include:
– Compressed Gas: Stored in high-pressure tanks (350–700 bar). Used in fuel cell vehicles.
– Liquid Hydrogen: Cooled to -253°C and stored in insulated tanks. Used in aerospace and some industrial applications.
– Chemical Carriers: Hydrogen is bound to liquids like ammonia or methanol for easier transport, then released at the point of use.
Pipelines are the most efficient way to move large volumes of hydrogen over long distances. Countries like the Netherlands and Germany are expanding their hydrogen pipeline networks, repurposing old natural gas lines.
The Future of Hydrogen Production
Scaling Up Green Hydrogen
The biggest hurdle for green hydrogen is cost. Currently, it’s two to three times more expensive than gray hydrogen. But as renewable energy prices fall and electrolyzer technology improves, costs are dropping fast. The International Energy Agency (IEA) predicts green hydrogen could reach $1–2 per kilogram by 2030 in sunny, windy regions.
Governments are stepping in to help. The U.S. Inflation Reduction Act offers tax credits of up to $3 per kilogram for clean hydrogen. The European Union’s REPowerEU plan aims to produce 10 million tons of renewable hydrogen domestically by 2030.
Hydrogen Hubs and Global Trade
To accelerate adoption, countries are building “hydrogen hubs”—integrated networks of production, storage, and use. These hubs connect renewable energy sources with industrial users, refueling stations, and export terminals.
For example, Australia is developing massive solar and wind farms in its outback to produce green hydrogen for export to Japan and South Korea. Chile is doing the same in its sun-drenched Atacama Desert. These projects could turn hydrogen into a global commodity, much like oil and gas today—but without the emissions.
Innovations on the Horizon
Researchers are exploring new frontiers in hydrogen production:
– Artificial Photosynthesis: Mimicking nature to produce hydrogen from sunlight and water using synthetic materials.
– Microbial Electrolysis: Using bacteria to enhance hydrogen production from organic waste.
– Plasma Reforming: Using high-energy plasma to break down methane or water into hydrogen with minimal emissions.
These technologies are still in early stages but could revolutionize how we make hydrogen in the coming decades.
Conclusion: A Cleaner Future Powered by Hydrogen
Hydrogen has the potential to transform our energy system—but only if it’s produced the right way. While steam methane reforming dominates today, the future belongs to green hydrogen made from water and renewable electricity. As technology improves and costs fall, we’re moving closer to a world where fuel cell vehicles, clean steel production, and zero-emission power generation become everyday realities.
The journey isn’t without challenges. We need better infrastructure, smarter policies, and continued innovation. But with global momentum building, hydrogen is no longer a niche idea—it’s a cornerstone of the clean energy transition. Whether you’re a policymaker, engineer, or just someone who cares about the planet, understanding how hydrogen is produced for fuel cells is a step toward a cleaner, more sustainable future.
Frequently Asked Questions
What is the most common method of producing hydrogen for fuel cells?
The most common method is steam methane reforming (SMR), which uses natural gas and steam to produce hydrogen. While efficient and cost-effective, it emits carbon dioxide unless paired with carbon capture technology.
What is green hydrogen and how is it made?
Green hydrogen is produced by electrolyzing water using electricity from renewable sources like wind or solar. This method emits no greenhouse gases and is considered the cleanest form of hydrogen production.
Can hydrogen be produced from renewable sources other than water?
Yes, hydrogen can also be made from biomass through gasification or from organic waste using microbial processes. These methods are sustainable but still under development or limited in scale.
Why is hydrogen purity important for fuel cells?
Fuel cells require high-purity hydrogen because impurities like carbon monoxide or sulfur can damage the catalyst and reduce efficiency. Most fuel cells need hydrogen that is 99.97% pure or higher.
Is hydrogen production expensive?
Currently, green hydrogen is more expensive than hydrogen from fossil fuels, but costs are falling rapidly due to cheaper renewables and improved electrolyzer technology. Government incentives are also helping to close the gap.
How is hydrogen transported and stored for use in fuel cells?
Hydrogen is typically stored as compressed gas in high-pressure tanks or as a liquid at very low temperatures. It can be transported via pipelines, trucks, or ships, often in chemical forms like ammonia for easier handling.

