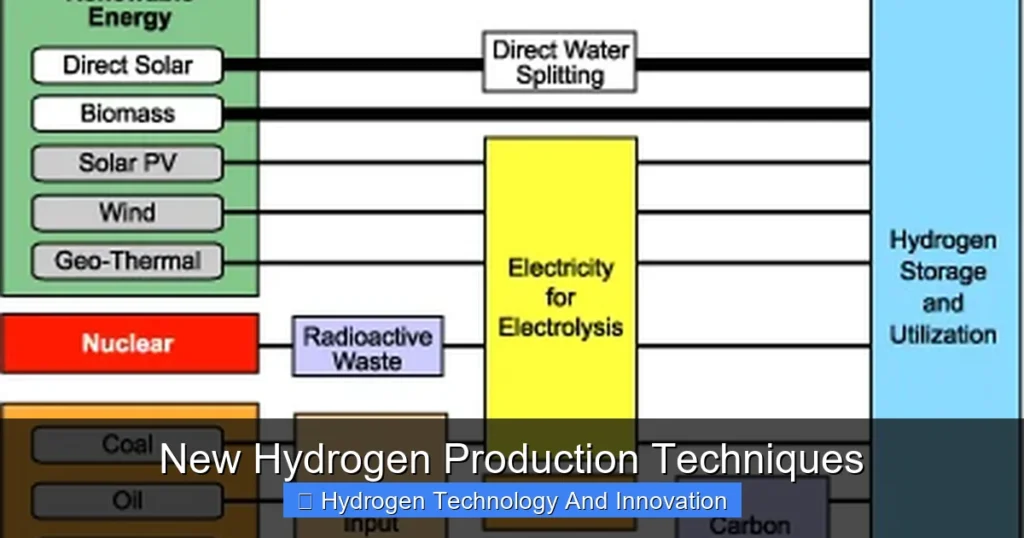
New hydrogen production techniques are revolutionizing clean energy by making hydrogen more sustainable, efficient, and affordable. Innovations like green electrolysis, thermochemical water splitting, and microbial production are paving the way for a hydrogen-powered future.
Key Takeaways
- Green hydrogen is now more viable: Advances in electrolysis powered by renewable energy are making green hydrogen cost-competitive with fossil-based methods.
- Solar-driven production is on the rise: Photocatalytic and photoelectrochemical systems use sunlight to split water, offering a direct path to clean hydrogen.
- Thermochemical cycles reduce emissions: High-temperature processes using nuclear or solar heat can produce hydrogen without greenhouse gases.
- Biological methods show promise: Microorganisms like algae and bacteria can generate hydrogen through fermentation or photosynthesis.
- Hybrid systems boost efficiency: Combining multiple technologies—like wind-powered electrolysis—maximizes output and reliability.
- Cost and scalability remain challenges: While innovations are impressive, widespread adoption depends on reducing costs and scaling infrastructure.
- Policy and investment are accelerating progress: Government support and private funding are driving rapid development in hydrogen tech.
📑 Table of Contents
- Introduction: The Hydrogen Revolution is Here
- Why Hydrogen Matters for a Clean Energy Future
- Green Hydrogen: The Gold Standard
- Solar-Powered Hydrogen: Harnessing the Sun Directly
- Thermochemical Hydrogen: Heat-Powered Production
- Biological Hydrogen: Nature’s Way
- Hybrid and Integrated Systems: The Best of All Worlds
- Challenges and the Road Ahead
- Conclusion: A Hydrogen-Powered Tomorrow
Introduction: The Hydrogen Revolution is Here
Imagine a world where the fuel that powers your car, heats your home, and runs factories emits nothing but water. That world is getting closer, thanks to exciting new hydrogen production techniques. Hydrogen has long been hailed as a clean energy carrier—its only byproduct when used is water vapor. But for decades, producing it cleanly and affordably has been a major hurdle. Most hydrogen today comes from natural gas through a process called steam methane reforming, which releases carbon dioxide. That’s changing fast.
Now, scientists and engineers are rolling out innovative methods to produce hydrogen without the carbon baggage. These new techniques are not just lab curiosities—they’re being tested in real-world projects and scaling up rapidly. From solar-powered water splitting to bacteria that churn out hydrogen, the field is buzzing with creativity and momentum. The goal? To make hydrogen a cornerstone of the global clean energy transition.
Why Hydrogen Matters for a Clean Energy Future

Visual guide about New Hydrogen Production Techniques
Image source: innovationnewsnetwork.com
Hydrogen isn’t just another energy source—it’s a versatile tool in the fight against climate change. Unlike batteries, which are great for short-term storage and small devices, hydrogen can store energy for weeks or months and power heavy industries like steelmaking, shipping, and aviation. It’s also a key ingredient in making synthetic fuels and fertilizers. But to unlock its full potential, we need to produce it cleanly.
The problem? Today, over 95% of hydrogen is “gray” hydrogen, made from fossil fuels. This process emits about 10 kilograms of CO₂ for every kilogram of hydrogen produced. That’s counterproductive if we’re trying to cut emissions. The solution lies in shifting to low-carbon or zero-carbon production methods. That’s where new hydrogen production techniques come in.
The Role of Policy and Investment
Governments around the world are stepping up. The U.S. Inflation Reduction Act offers generous tax credits for clean hydrogen production. The European Union has its Hydrogen Strategy, aiming for 10 million tons of renewable hydrogen by 2030. Japan and South Korea are investing heavily in hydrogen infrastructure. These policies are creating a fertile ground for innovation.
Private companies are also jumping in. Startups like Hysata and Enapter are developing ultra-efficient electrolyzers. Oil giants like Shell and BP are launching green hydrogen projects. Even automakers like Toyota and Hyundai are betting big on hydrogen fuel cells. This surge in interest is driving down costs and speeding up deployment.
Environmental and Economic Benefits
Clean hydrogen can decarbonize sectors that are hard to electrify. Think long-haul trucks, cargo ships, and industrial furnaces. It can also balance the grid by storing excess renewable energy. On windy or sunny days, when solar and wind generate more power than needed, that extra electricity can be used to make hydrogen. Then, during calm or cloudy periods, the hydrogen can be converted back to electricity or used directly.
Economically, hydrogen can create jobs and revitalize industrial regions. Ports could become hydrogen hubs, exporting clean fuel globally. Rural areas with abundant wind or solar could become energy exporters. The International Energy Agency estimates that hydrogen could support 30 million jobs by 2050.
Green Hydrogen: The Gold Standard
When people talk about clean hydrogen, they’re usually referring to “green hydrogen”—hydrogen made using renewable electricity to split water into hydrogen and oxygen. This process, called electrolysis, has been around for over a century. But recent advances are making it faster, cheaper, and more efficient.
How Electrolysis Works
Electrolysis uses an electric current to break water (H₂O) into hydrogen (H₂) and oxygen (O₂). The device that does this is called an electrolyzer. There are three main types: alkaline, proton exchange membrane (PEM), and solid oxide electrolyzers. Each has its strengths.
Alkaline electrolyzers are mature and cost-effective but less flexible. PEM systems are more responsive and compact, making them ideal for pairing with variable renewables like wind and solar. Solid oxide electrolyzers operate at high temperatures and are highly efficient, especially when paired with industrial heat or nuclear power.
Breakthroughs in Electrolyzer Technology
One of the biggest challenges has been cost. Electrolyzers have been expensive to build and maintain. But new materials and designs are changing that. For example, researchers are developing non-precious metal catalysts to replace expensive platinum and iridium. These catalysts can reduce costs by up to 50%.
Another breakthrough is in efficiency. Traditional electrolyzers convert about 60–70% of electrical energy into hydrogen. New designs are pushing that to 80% or higher. Hysata, an Australian startup, claims its capillary-fed electrolyzer achieves 95% efficiency—close to the theoretical maximum. That means less energy wasted and lower operating costs.
Scaling Up with Renewable Energy
The real magic happens when electrolysis is powered by renewables. Wind farms in the North Sea, solar arrays in the Sahara, and hydropower in Canada are all being used to produce green hydrogen. In Australia, the Asian Renewable Energy Hub plans to use 26 gigawatts of wind and solar to make hydrogen for export.
These projects are not just about energy—they’re about economic transformation. Remote areas with abundant clean power can become hydrogen exporters, creating new revenue streams. Countries like Chile, Namibia, and Saudi Arabia are positioning themselves as future hydrogen superpowers.
Solar-Powered Hydrogen: Harnessing the Sun Directly
What if we could make hydrogen directly from sunlight, without needing electricity first? That’s the promise of solar-driven hydrogen production. These methods use light to trigger chemical reactions that split water, mimicking photosynthesis in plants.
Photocatalytic Water Splitting
Photocatalysis uses semiconductor materials that absorb sunlight and generate electrons. These electrons then drive the water-splitting reaction. Titanium dioxide is a common photocatalyst, but it only works with ultraviolet light, which makes up just 5% of sunlight. Researchers are developing new materials that can use visible light—up to 45% of the solar spectrum.
For example, scientists at the University of Cambridge have created a catalyst made of carbon nitride and graphene that efficiently produces hydrogen under sunlight. The challenge? Scaling up and improving durability. Most photocatalysts degrade over time, reducing efficiency.
Photoelectrochemical (PEC) Cells
PEC cells combine a light-absorbing material with an electrolyte to produce hydrogen directly. Think of it as a solar panel that makes fuel instead of electricity. These systems can be more efficient than photocatalysis because they separate the light absorption and reaction processes.
The National Renewable Energy Laboratory (NREL) in the U.S. has developed a PEC cell that operates at over 10% solar-to-hydrogen efficiency. That’s still lower than electrolysis, but PEC systems have the advantage of simplicity—no need for separate solar panels and electrolyzers.
Artificial Photosynthesis: The Ultimate Goal
The holy grail is artificial photosynthesis—a system that mimics how plants convert sunlight, water, and CO₂ into energy. Researchers are working on “artificial leaves” that float on water and produce hydrogen when exposed to sunlight. These devices could be deployed on reservoirs, oceans, or even rooftops.
While still in early stages, artificial photosynthesis could revolutionize hydrogen production. It would eliminate the need for large solar farms and complex infrastructure. Imagine a future where every home has a small device that makes hydrogen from rainwater and sunlight.
Thermochemical Hydrogen: Heat-Powered Production
Not all new hydrogen production techniques rely on electricity. Some use heat—especially high-temperature heat from nuclear reactors or concentrated solar power—to drive chemical reactions that produce hydrogen.
Thermochemical Water Splitting
This process uses a series of chemical reactions, powered by heat, to split water. Unlike electrolysis, it doesn’t require electricity. Instead, it cycles through metal oxides or sulfur-based compounds that release oxygen and then react with water to produce hydrogen.
One well-known cycle is the sulfur-iodine (S-I) process, developed by General Atomics. It operates at temperatures around 850°C and can achieve high efficiency. The heat can come from nuclear reactors or solar concentrators.
Nuclear-Powered Hydrogen
Nuclear energy is a natural fit for thermochemical hydrogen. Reactors like the High-Temperature Gas-Cooled Reactor (HTGR) can provide the steady, high-temperature heat needed. The U.S. Department of Energy is funding projects to integrate nuclear plants with hydrogen production.
For example, the Idaho National Laboratory is testing a system that uses nuclear heat to produce hydrogen via thermochemical cycles. This could provide a clean, reliable source of hydrogen for industrial use, even when the sun isn’t shining or the wind isn’t blowing.
Solar Thermal Hydrogen
Concentrated solar power (CSP) plants use mirrors to focus sunlight and generate heat. This heat can drive thermochemical reactions. In Australia, the CSIRO is testing a solar thermochemical reactor that produces hydrogen at 1,000°C.
These systems are still experimental, but they offer a way to store solar energy as chemical fuel. The hydrogen can be used on-site or transported to where it’s needed.
Biological Hydrogen: Nature’s Way
Nature has been making hydrogen for billions of years. Now, scientists are tapping into that power. Biological hydrogen production uses microorganisms like algae, bacteria, and fungi to generate hydrogen through natural processes.
Algae and Cyanobacteria
Certain types of green algae and cyanobacteria can produce hydrogen under specific conditions. When deprived of sulfur, some algae switch from producing oxygen to producing hydrogen. This process, called photobiological hydrogen production, uses sunlight and water.
Researchers at the University of Turku in Finland have engineered algae strains that produce hydrogen more efficiently. The challenge is keeping the process stable—hydrogen production often stops after a few hours.
Dark Fermentation
Some bacteria produce hydrogen as a byproduct of breaking down organic matter in the absence of oxygen. This is called dark fermentation. It can use waste materials like food scraps, agricultural residues, or sewage sludge as feedstock.
The advantage? It turns waste into energy. A pilot plant in Spain uses dark fermentation to produce hydrogen from olive mill wastewater. The process also reduces pollution and generates biogas as a bonus.
Microbial Electrolysis Cells (MECs)
MECs combine biology and electrochemistry. Bacteria break down organic matter and produce electrons, which are then used to generate hydrogen at a cathode. Only a small amount of electricity is needed—much less than in traditional electrolysis.
These systems are still in the lab, but they could be ideal for treating wastewater while producing clean fuel. Imagine a sewage treatment plant that powers itself with hydrogen.
Hybrid and Integrated Systems: The Best of All Worlds
The future of hydrogen production isn’t about picking one method—it’s about combining them. Hybrid systems integrate multiple technologies to boost efficiency, reliability, and cost-effectiveness.
Wind + Electrolysis
One of the most common hybrids pairs wind turbines with electrolyzers. When the wind blows, excess power is used to make hydrogen. This solves the problem of curtailment—when turbines are turned off because the grid can’t absorb the power.
In Denmark, the HySynergy project uses offshore wind to produce green hydrogen for industrial use. The system includes storage and a fueling station for hydrogen trucks.
Solar + Storage + Electrolysis
Solar farms often generate power during the day, but demand peaks in the evening. By adding batteries and electrolyzers, excess solar can be stored as hydrogen. Then, during peak hours, the hydrogen can be converted back to electricity using fuel cells.
In California, the HelioSage project combines solar panels, batteries, and electrolyzers to provide round-the-clock clean energy. This approach is especially useful in remote areas without grid access.
Nuclear + Thermochemical + Electrolysis
Some advanced systems use nuclear heat for thermochemical cycles and electricity for electrolysis. This dual approach maximizes hydrogen output and provides flexibility. The heat can be used for industrial processes, while the hydrogen supports transportation and grid storage.
Challenges and the Road Ahead
Despite the excitement, new hydrogen production techniques face real challenges. Cost is still a barrier. Green hydrogen currently costs $3–$6 per kilogram, compared to $1–$2 for gray hydrogen. While costs are falling, they need to drop further to compete.
Infrastructure is another hurdle. We need pipelines, storage facilities, and refueling stations. Building this network will take time and investment. Safety is also a concern—hydrogen is flammable and requires careful handling.
But the momentum is undeniable. As technology improves and economies of scale kick in, hydrogen is becoming more affordable. The Hydrogen Council predicts that green hydrogen could cost less than $2 per kilogram by 2030.
Conclusion: A Hydrogen-Powered Tomorrow
The era of dirty hydrogen is ending. New hydrogen production techniques are making clean, sustainable hydrogen a reality. From solar-powered water splitting to bacteria that eat waste and spit out fuel, the innovations are as diverse as they are exciting.
These technologies aren’t just scientific achievements—they’re tools for building a cleaner, fairer, and more resilient energy system. With the right policies, investments, and public support, hydrogen can play a central role in achieving net-zero emissions.
The journey won’t be easy, but the destination is worth it. A world powered by clean hydrogen is not a distant dream—it’s within reach. And with every breakthrough, we get one step closer.
Frequently Asked Questions
What is green hydrogen?
Green hydrogen is hydrogen produced by splitting water using renewable electricity, such as wind or solar power. It emits no carbon dioxide during production, making it the cleanest form of hydrogen.
How efficient are new hydrogen production methods?
Efficiency varies by method. Electrolysis typically achieves 60–80% efficiency, while advanced systems like Hysata’s claim up to 95%. Solar-driven methods are less efficient (5–15%) but are improving rapidly.
Can hydrogen be produced from waste?
Yes, through processes like dark fermentation and microbial electrolysis. Bacteria can break down organic waste and produce hydrogen as a byproduct, turning trash into clean energy.
Is hydrogen production safe?
Hydrogen is flammable and requires proper handling, but with modern safety systems, it can be stored and transported safely. Industry standards and regulations are continually improving.
How long until green hydrogen is cost-competitive?
Experts predict green hydrogen could cost less than $2 per kilogram by 2030, thanks to falling renewable energy prices and improved electrolyzer technology.
Can I produce hydrogen at home?
Small-scale electrolyzers are available for home use, but they’re currently expensive and inefficient. Most home hydrogen production is experimental, though future systems may become more practical.