Hydrogen fuel cells generate clean electricity by combining hydrogen and oxygen, producing only water and heat as byproducts. This innovative technology powers vehicles, homes, and industries without harmful emissions, offering a sustainable alternative to fossil fuels.
Key Takeaways
- Hydrogen fuel cells produce electricity through an electrochemical reaction, not combustion. This means no greenhouse gases are released during operation.
- The core components include an anode, cathode, electrolyte, and catalyst. These parts work together to split hydrogen atoms and generate an electric current.
- Fuel cells are highly efficient, often exceeding 60% energy conversion. That’s significantly higher than traditional internal combustion engines.
- They are used in cars, buses, forklifts, and backup power systems. Real-world applications are growing rapidly across transportation and industry.
- Hydrogen must be produced and stored safely. Most hydrogen today comes from natural gas, but green hydrogen from renewable sources is on the rise.
- Refueling is fast—similar to gasoline—taking just 3–5 minutes. This makes hydrogen vehicles practical for long-distance travel.
- Challenges include cost, infrastructure, and hydrogen production methods. Ongoing innovation aims to make fuel cells more affordable and sustainable.
📑 Table of Contents
Introduction to Hydrogen Fuel Cells
Imagine a world where cars run on water, buses emit only clean air, and homes are powered without burning fossil fuels. That future is closer than you think—thanks to hydrogen fuel cells. These remarkable devices generate electricity through a clean, quiet process that produces zero harmful emissions. Unlike batteries that need recharging, fuel cells can keep producing power as long as they have a supply of hydrogen and oxygen.
Hydrogen fuel cells are not science fiction. They’ve been used in space missions since the 1960s and are now making their way into everyday life. From Toyota’s Mirai to hydrogen-powered forklifts in warehouses, this technology is proving its worth. But how exactly do they work? At their core, fuel cells are like batteries—but with a twist. Instead of storing energy, they create it on demand using hydrogen gas. The result? A reliable, efficient, and environmentally friendly power source.
The Science Behind Hydrogen Fuel Cells
To understand how hydrogen fuel cells work, let’s break it down into simple steps. At its most basic level, a fuel cell uses hydrogen and oxygen to produce electricity, water, and a bit of heat. No combustion, no smoke, no pollution. It’s all done through a quiet electrochemical reaction—a process that happens silently inside the cell.
Visual guide about How Hydrogen Fuel Cells Work
Image source: volvoceblog.com
Basic Components of a Fuel Cell
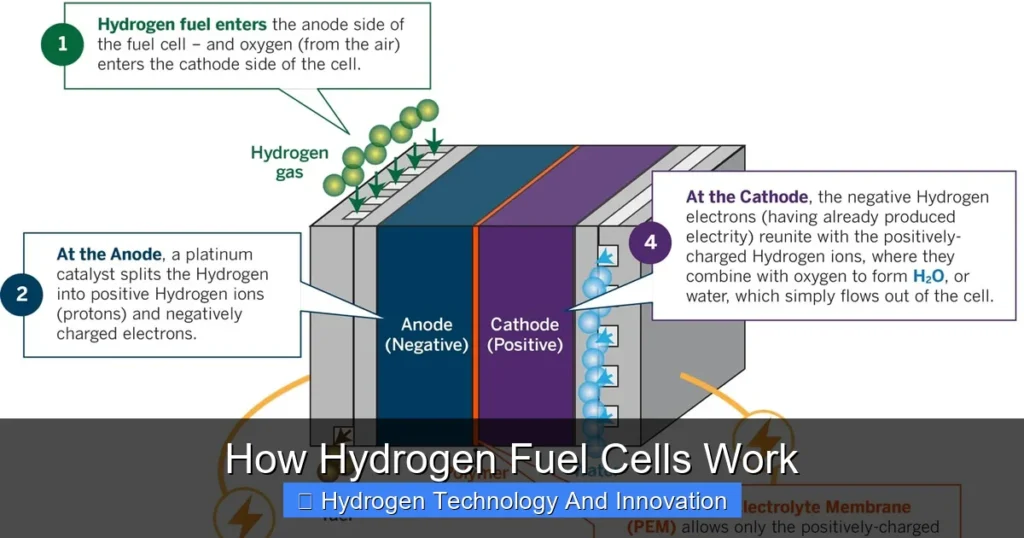
Every hydrogen fuel cell has four main parts: the anode, cathode, electrolyte, and catalyst. Think of them as the ingredients in a recipe for clean energy. The anode is the negative side where hydrogen gas enters. The cathode is the positive side where oxygen (usually from the air) is introduced. Between them lies the electrolyte, a special material that allows only certain particles to pass through. Finally, the catalyst—often made of platinum—speeds up the chemical reactions.
The Electrochemical Reaction
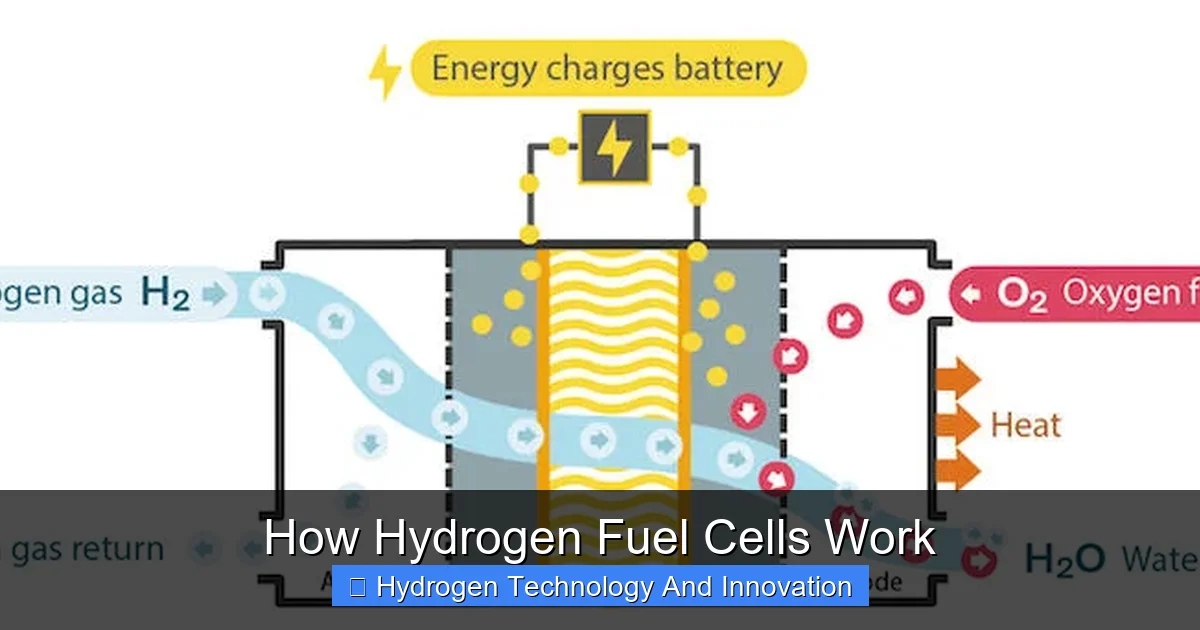
Here’s where the magic happens. When hydrogen gas (H₂) reaches the anode, the catalyst splits each hydrogen molecule into two protons and two electrons. The protons move through the electrolyte to the cathode, while the electrons are forced to travel through an external circuit—creating an electric current. This current can power a motor, light a bulb, or charge a battery.
At the cathode, the protons, electrons, and oxygen from the air combine to form water (H₂O). That’s it! The only byproducts are pure water and a small amount of heat. No carbon dioxide, no nitrogen oxides, no soot. Just clean energy and a few drops of water.
Types of Fuel Cells
Not all fuel cells are the same. There are several types, each designed for different uses. The most common for vehicles and portable power is the Proton Exchange Membrane (PEM) fuel cell. It operates at relatively low temperatures (around 80°C), starts quickly, and is compact—perfect for cars and buses.
Other types include Solid Oxide Fuel Cells (SOFC), which run at high temperatures and are great for stationary power generation, and Alkaline Fuel Cells (AFC), used in space missions. Each has its strengths, but PEM fuel cells are leading the charge in transportation due to their efficiency and responsiveness.
How Hydrogen Fuel Cells Generate Electricity
Let’s walk through the step-by-step process of how a hydrogen fuel cell generates electricity. It’s a bit like a water wheel, but instead of flowing water, it’s the flow of electrons that does the work.
Step 1: Hydrogen Enters the Anode
Hydrogen gas is fed into the anode side of the fuel cell. This hydrogen can come from a tank on a vehicle or a stationary storage system. The gas molecules (H₂) are under pressure, ensuring a steady flow into the cell.
Step 2: Catalyst Splits Hydrogen Atoms
At the anode, a platinum-based catalyst breaks each hydrogen molecule into two protons (H⁺) and two electrons (e⁻). This is called oxidation. The protons are positively charged and can move through the electrolyte, but the electrons cannot. They’re forced to take a different path.
Step 3: Electrons Create Electric Current
The electrons travel through an external circuit—like a wire—to reach the cathode. This flow of electrons is what we call electricity. It can power a motor, charge a battery, or run appliances. The more electrons that flow, the more power is generated.
Step 4: Protons Move Through the Electrolyte
While the electrons are busy powering devices, the protons move through the electrolyte membrane toward the cathode. The electrolyte is designed to be selective—only allowing protons to pass. This separation is crucial for the reaction to continue.
Step 5: Oxygen Reacts at the Cathode
At the cathode, oxygen from the air (O₂) meets the arriving protons and electrons. They combine to form water (H₂O). This is called reduction. The water then exits the cell, usually as vapor or liquid, depending on the temperature.
Step 6: Continuous Power Generation
As long as hydrogen and oxygen are supplied, the reaction continues. The fuel cell doesn’t “run out” like a battery. It just needs a constant fuel source. This makes it ideal for applications where long runtime and quick refueling are important—like in delivery trucks or emergency power systems.
Applications of Hydrogen Fuel Cells
Hydrogen fuel cells are versatile. They’re not just for cars—they’re being used in a wide range of industries and settings. Let’s explore some of the most exciting applications.
Transportation: Cars, Buses, and Trucks
One of the biggest uses of hydrogen fuel cells is in transportation. Companies like Toyota, Hyundai, and Honda have launched hydrogen-powered vehicles. The Toyota Mirai, for example, can travel over 400 miles on a single tank and refuels in under five minutes—much faster than charging an electric car.
Buses are another success story. Cities like London, Tokyo, and Los Angeles are testing hydrogen buses. They’re quiet, emit only water, and can operate all day without recharging. Heavy-duty trucks are also getting in on the action. Companies like Nikola and Hyundai are developing hydrogen-powered semis for long-haul freight, where battery weight and charging time are major hurdles.
Material Handling: Forklifts and Warehouse Equipment
Inside warehouses, hydrogen fuel cells are revolutionizing material handling. Forklifts powered by fuel cells can run for a full shift on one tank of hydrogen. Refueling takes just a few minutes—no need to swap out heavy batteries. This increases productivity and reduces downtime.
Amazon and Walmart have deployed thousands of hydrogen forklifts in their distribution centers. The result? Cleaner air, lower operating costs, and faster operations. Plus, fuel cells perform well in cold storage environments, where batteries lose efficiency.
Stationary Power: Backup and Primary Energy
Fuel cells are also used for stationary power. They can provide backup electricity for hospitals, data centers, and emergency shelters. Unlike diesel generators, they’re quiet, emissions-free, and can run for days with a steady hydrogen supply.
In some cases, fuel cells serve as the primary power source. For example, in remote areas or islands, hydrogen systems can replace diesel generators. They’re also being integrated into microgrids—small, local energy networks that can operate independently from the main grid.
Marine and Aviation: The Future of Clean Travel
The maritime and aviation industries are exploring hydrogen fuel cells for cleaner travel. Ships powered by hydrogen could reduce ocean pollution, while hydrogen-powered planes might one day offer zero-emission flights. Companies like Airbus are working on hydrogen aircraft concepts, aiming for commercial use by 2035.
Hydrogen Production and Storage
For fuel cells to be truly clean, the hydrogen they use must be produced sustainably. Right now, most hydrogen comes from natural gas through a process called steam methane reforming. This method releases carbon dioxide, so it’s not ideal for the environment.
Green Hydrogen: The Clean Alternative
The future lies in green hydrogen—hydrogen produced using renewable energy. Electrolysis splits water (H₂O) into hydrogen and oxygen using electricity from solar, wind, or hydro power. If the electricity is clean, the hydrogen is too. This is the key to a truly sustainable fuel cell economy.
Countries like Germany, Australia, and Japan are investing heavily in green hydrogen projects. Iceland already produces most of its hydrogen this way. As renewable energy becomes cheaper, green hydrogen will become more competitive.
Storing and Transporting Hydrogen
Hydrogen is tricky to store. It’s the lightest element and can leak easily. It’s usually stored as a compressed gas in high-pressure tanks or as a liquid at very low temperatures (-253°C). Both methods require strong, lightweight materials and careful handling.
Transporting hydrogen is another challenge. It can be moved via pipelines, trucks, or ships—but infrastructure is still limited. Building a hydrogen economy will require new pipelines, refueling stations, and safety standards. Governments and companies are working together to expand this network.
Advantages and Challenges of Hydrogen Fuel Cells
Hydrogen fuel cells offer many benefits, but they’re not without challenges. Let’s look at both sides of the coin.
Advantages
- Zero Emissions: Only water and heat are produced during operation.
- High Efficiency: Fuel cells convert 60% or more of hydrogen’s energy into electricity—double that of gasoline engines.
- Fast Refueling: Takes 3–5 minutes, similar to gasoline.
- Quiet Operation: No engine noise, making them ideal for urban areas.
- Long Range: Hydrogen vehicles can travel 300–400 miles on a single tank.
Challenges
- High Cost: Fuel cells and hydrogen production are still expensive. Platinum catalysts and storage tanks drive up prices.
- Limited Infrastructure: Few hydrogen refueling stations exist, especially outside major cities.
- Hydrogen Production: Most hydrogen today is not green, undermining environmental benefits.
- Storage and Safety: Hydrogen is flammable and requires careful handling.
Despite these hurdles, progress is being made. Researchers are developing cheaper catalysts, better storage methods, and more efficient production techniques. Governments are offering incentives, and companies are investing in hydrogen technology.
The Future of Hydrogen Fuel Cells
The future of hydrogen fuel cells looks bright. As the world shifts toward clean energy, hydrogen is emerging as a key player. It’s not meant to replace batteries—but to complement them. While batteries are great for short trips and small devices, hydrogen excels in heavy-duty, long-range applications.
Experts predict that hydrogen could supply 10–20% of global energy needs by 2050. It could power trucks, ships, planes, and even entire cities. With continued innovation, hydrogen fuel cells could become as common as gasoline engines—but without the pollution.
The journey isn’t easy, but the payoff is huge. Clean air, energy independence, and a healthier planet are worth the effort. The next time you see a hydrogen-powered bus or hear about a new refueling station, remember: you’re witnessing the dawn of a cleaner energy era.
Frequently Asked Questions
How do hydrogen fuel cells produce electricity?
Hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. Hydrogen is split into protons and electrons at the anode, and the electrons flow through a circuit to create current, while protons move to the cathode to form water.
Are hydrogen fuel cells safe?
Yes, hydrogen fuel cells are safe when handled properly. Hydrogen is flammable, but modern systems include safety features like leak detectors, pressure relief valves, and strong storage tanks. Millions of hours of operation in vehicles and facilities show a strong safety record.
What are the main byproducts of a hydrogen fuel cell?
The only byproducts are water and a small amount of heat. No carbon dioxide, nitrogen oxides, or particulate matter are emitted during operation, making fuel cells a clean energy source.
How long does it take to refuel a hydrogen vehicle?
Refueling a hydrogen vehicle takes about 3 to 5 minutes, similar to filling up a gasoline car. This is much faster than charging an electric vehicle, making hydrogen ideal for long-distance travel.
Can hydrogen fuel cells be used in homes?
Yes, hydrogen fuel cells can provide electricity and heat for homes. They’re used in residential fuel cell systems in countries like Japan and Germany, offering reliable, clean power and reducing reliance on the grid.
Is hydrogen fuel more expensive than gasoline?
Currently, hydrogen fuel is more expensive than gasoline, but costs are falling. As production scales up and green hydrogen becomes more common, prices are expected to drop, making it competitive with traditional fuels.